A combination of metabolic labeling and 2D-DIGE analysis in response to a farnesyltransferase inhibitor facilitates the discovery of new prenylated proteins†
Abstract
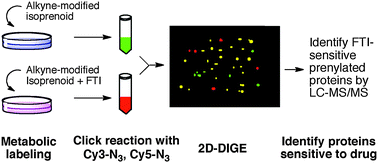
Protein prenylation is a post-translational modification required for proper cellular localization and activity of many important eukaryotic proteins. Farnesyltransferase inhibitors (FTIs) have been explored extensively for their antitumor activity. To assist in identifying potentially new and more useful markers for therapeutic applications, we developed a strategy that uses a combination of metabolic labeling and 2D DIGE (differential gel electrophoresis) to discover new prenylated proteins whose cellular levels are influenced by FTIs. In this approach, metabolic labeling of prenylated proteins was first carried out with an alkyne-modified isoprenoid analog, C15Alk, in the presence or absence of the FTI L-744,832. The resulting alkyne-tagged proteins were then labeled with Cy3-N3 and Cy5-N3 and subjected to 2D-DIGE. Multiple spots having altered levels of labeling in presence of the FTI were observed. Mass spectrometric analysis of some of the differentially labeled spots identified several known prenylated proteins, along with HisRS, PACN-3, GNAI-1 and GNAI-2, which are not known to be prenylated. In vitro farnesylation of a C-terminal peptide sequence derived from GNAI-1 and GNAI-2 produced a farnesylated product, suggesting GNAI-1 and GNAI-2 are potential novel farnesylated proteins. These results suggest that this new strategy could be useful for the identification of prenylated proteins whose level of post-translational modification has been modulated by the presence of an FTI. Additionally, this approach, which decreases sample complexity and thereby facilitates analysis, should be applicable to studies of other post-translational modifications as well.

 Please wait while we load your content...
Please wait while we load your content...