Molecular docking and molecular dynamics studies on β-lactamases and penicillin binding proteins
Abstract
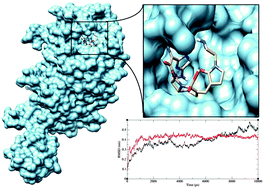
Bacterial resistance to β-lactam antibiotics poses a serious threat to human health. Penicillin binding proteins (PBPs) and β-lactamases are involved in both antibacterial activity and mediation of β-lactam antibiotic resistance. The two major reasons for resistance to β-lactams include: (i) pathogenic bacteria expressing drug insensitive PBPs rendering β-lactam antibiotics ineffective and (ii) production of β-lactamases along with alteration of their specificities. Thus, there is an urgent need to develop newer β-lactams to overcome the challenge of bacterial resistance. Therefore the present study aims to identify the binding affinity of β-lactam antibiotics with different types of PBPs and β-lactamases. In this study, cephalosporins and carbapenems are docked into PBP2a of Staphylococcus aureus, PBP2b and PBP2x of Streptococcus pneumoniae and SHV-1 β-lactamase of Escherichia coli. The results reveal that Ceftobiprole can efficiently bind to PBP2a, PBP2b and PBP2x and not strongly to SHV-1 β-lactamase. Furthermore, molecular dynamics (MD) simulations are performed to refine the binding mode of the docked complex structure and to observe the differences in the stability of free PBP2x and Ceftobiprole bound PBP2x. MD simulation supports the greater stability of the Ceftobiprole–PBP2x complex compared to free PBP2x. This work demonstrates that potential β-lactam antibiotics can efficiently bind to different types of PBPs for circumventing β-lactam resistance and opens avenues for the development of newer antibiotics that can target bacterial pathogens.

 Please wait while we load your content...
Please wait while we load your content...