Thermodynamics of interfacial changes in a protein–protein complex
Abstract
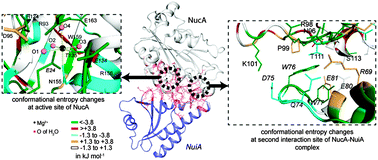
Recent experiments with biomacromolecular complexes suggest that structural modifications at the interfaces are vital for stability of the complexes and the functions of the biomacromolecules. Although several qualitative aspects about such interfaces are known from structural data, quantification of the interfacial changes is lacking. In this work, we study the thermodynamic changes at the interface in the complex between an enzyme, Nuclease A (NucA), and a specific inhibitor protein, NuiA. We calculate the conformational free energy and conformational entropy costs from histograms of the dihedral angles generated from all-atom molecular dynamics simulations on the complex and the free proteins. We extract the conformational thermodynamic parameters for changes in the tertiary structure of NuiA. We show that the binding is dominated by the interfacial changes, where the basic residues of NucA and acidic residues of NuiA are highly ordered and stabilized via strong electrostatic interactions. Our results correlate well with known information from structural studies. The tight interfacial structure is reflected in the significant changes in the structure and dynamics of the water molecules at the enzyme–inhibitor interface. The interfacial water molecules contribute significantly to the entropy loss for the overall complexation.

 Please wait while we load your content...
Please wait while we load your content...