Sequential glycan profiling at single cell level with the microfluidic lab-in-a-trench platform: a new era in experimental cell biology†
Abstract
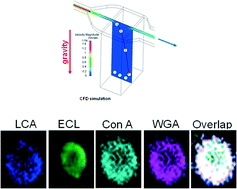
It is now widely recognised that the earliest changes that occur on a cell when it is stressed or becoming diseased are alterations in its surface glycosylation. Current state-of-the-art technologies in glycoanalysis include mass spectrometry, protein microarray formats, techniques in cytometry and more recently, glyco-quantitative polymerase chain reaction (Glyco-qPCR). Techniques for the glycoprofiling of the surfaces of single cells are either limited to the analysis of large cell populations or are unable to handle multiple and/or sequential probing. Here, we report a novel approach of single live cell glycoprofiling enabled by the microfluidic “Lab-in-a-Trench” (LiaT) platform for performing capture and retention of cells, along with shear-free reagent loading and washing. The significant technical improvement on state-of-the-art is the demonstration of consecutive, spatio-temporally profiling of glycans on a single cell by sequential elution of the previous lectin probe using their corresponding free sugar. We have qualitatively analysed glycan density on the surface of individual cells. This has allowed us to qualitatively co-localise the observed glycans. This approach enables exhaustive glycoprofiling and glycan mapping on the surface of individual live cells with multiple lectins. The possibility of sequentially profiling glycans on cells will be a powerful new tool to add to current glycoanalytical techniques. The LiaT platform will enable cell biologists to perform many high sensitivity assays and also will also make a significant impact on biomarker research.

 Please wait while we load your content...
Please wait while we load your content...