PDMS based coplanar microfluidic channels for the surface reduction of oxidized Galinstan†
Abstract
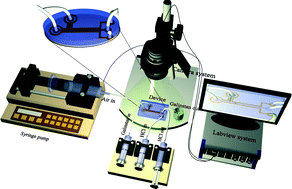
Galinstan has the potential to replace mercury – one of the most popular liquid metals. However, the easy oxidation of Galinstan restricts wide applicability of the material. In this paper, we report an effective reduction method for the oxidized Galinstan using gas permeable PDMS (polydimethlysiloxane)-based microfluidic channel. The complete study is divided into two parts – reduction of Galinstan oxide and behavior of reduced Galinstan oxide in a microfluidic channel. The reduction of Galinstan oxide is discussed on the basis of static as well as dynamic angles. The contact angle analyses help to find the extent of reduction by wetting characteristics of the oxide, to optimize PDMS thickness and to select suitable hydrochloric acid (HCl) concentration. The highest advancing angle of 155° and receding angle of 136° is achieved with 200 μm thick PDMS film and 37 wt% (weight percent) HCl solution. The behavior of reduced Galinstan oxide is analyzed in PDMS-based coplanar microfluidic channels fabricated using a simple micromolding technique. Galinstan in the microfluidic channel is surrounded by another coplanar channel filled with HCl solution. Due to the excellent permeability of PDMS, HCl permeates through the PDMS wall between the two channels (interchannel PDMS wall) and achieves a continuous chemical reaction with oxidized Galinstan. A Lab VIEW controlled syringe pump is used for observing the behavior of HCl treated Galinstan in the microfluidic channel. Further optimization of the microfluidic device has been conducted to minimize the reoxidation of reduced Galinstan oxide in the microfluidic channel.

 Please wait while we load your content...
Please wait while we load your content...