Speciation analysis of sugar phosphates via anion exchange chromatography combined with inductively coupled plasma dynamic reaction cell mass spectrometry – optimization for the analysis of yeast cell extracts
Abstract
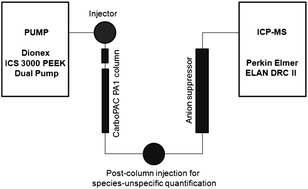
Anion exchange chromatography combined with inductively coupled plasma mass spectrometry was introduced and optimized for the separation and accurate quantification of sugar phosphates in cell extracts. Sugar phosphates have been separated on an anion exchange quaternary ammonium functionalized stationary phase (Dionex CarboPAC PA1) via gradient elution with 160 mM Na2CO3 and 50 mM NaOH. After separation, the sugar phosphates were on-line transferred to an inductively coupled plasma mass spectrometer after having passed an anion self-regenerating suppressor. Detection was performed via phosphorus as PO+ (m/z 47) was generated by the dynamic reaction cell technique using oxygen as a reaction gas. The ionization suppression process in the inductively coupled plasma caused by the high buffer strength of the chromatographic gradient was assessed by post-column flow injection analysis. It could be shown that the anion self-regenerating suppressor led to a significant reduction of signal suppression. With the new methodology excellent relative standard deviations of retention times of sugar phosphates for short- and long-term measurements were achieved. The relative standard deviations of short-term and long-term repeatability of peak areas were below 3.0 and 10.0% respectively. The absolute on-column LODs and LOQs of the optimized method were in the sub-picomole range. The developed method was applied for the purity assessment of commercially available sugar phosphate standards and evaluated for the quantification of sugar phosphates in yeast samples (Pichia pastoris) after extraction with boiling ethanol. Due to the lack of reference materials, the method accuracy was validated by method inter-comparison with an LCxLC-ESI-MS/MS based method, which has been developed in our laboratory.

 Please wait while we load your content...
Please wait while we load your content...