Protein synthesis yield increased 72 times in the cell-free PURE system†
Abstract
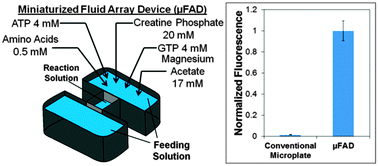
Compared to cell-based protein expression, cell-free protein synthesis (CFPS) offers several advantages including a greater control over system additives. This control is further enhanced with a CFPS system called the Protein synthesis Using Recombinant Elements (PURE) system, which consists of 108 purified transcriptional and translational elements. With the PURE system, all elements are known, nuclease and protease activities are reduced, and the concentration of each element can be optimized for maximal protein expression. However, protein expression yield with this system is relatively low due to the consumption of nutrients and energy molecules as well as the accumulation of inhibitory byproducts in the batch format. To enhance protein expression with the PURE system, we developed a feeding solution that was optimized using a miniaturized fluid array device (μFAD) in a continuous-exchange cell-free (CECF) format. The device enabled (1) continuous supply of energy/nutrient molecules from the feeding solution to the reaction solution where protein synthesis occurred, and (2) simultaneous removal of inhibitory expression byproducts from the reaction solution to the feeding solution. Consequently, the synthesis yield of green fluorescent protein (GFP) increased 72.5-fold in comparison with the same reaction in the conventional batch format.

 Please wait while we load your content...
Please wait while we load your content...