Biodegradability of 27 pyrrolidinium, morpholinium, piperidinium, imidazolium and pyridinium ionic liquid cations under aerobic conditions†
Abstract
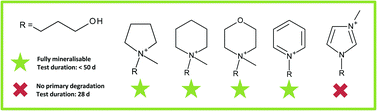
The chemical and thermal stability of ionic liquids (ILs) makes them interesting for a large variety of applications in nearly all areas of the chemical industry. However, this stability is often reflected in their recalcitrance towards biodegradation, which comes with the risk of persistence when they are released into the environment. In this study we carried out a systematic investigation of the biodegradability of pyrrolidinium, morpholinium, piperidinium, imidazolium and pyridinium-based IL cations substituted with different alkyl or functionalised side chains and using halide counterions. We examined their primary degradability by specific analysis and/or their ultimate biodegradability using biochemical oxygen demand tests according to OECD guideline 301F. Biological transformation products were investigated using mass spectrometry. A comparison of the biodegradation potential of these ILs shows that for all five head groups, representatives can be found that are readily or inherently biodegradable, thus permitting the structural design of ILs with a reduced environmental hazard.

 Please wait while we load your content...
Please wait while we load your content...