Selective isomerization–carbonylation of a terpene trisubstituted double bond†
Abstract
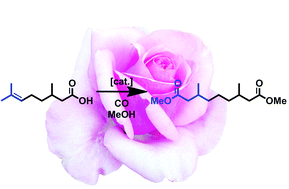
Selective catalytic carbonylation of the trisubstituted double bond of citronellic acid is enabled via an isomerization–functionalization approach. Alkoxycarbonylation with [{1,2-(tBu2PCH2)2C6H4}Pd(OTf)2] as a catalyst precursor occurs with >97% selectivity for the terminal diester dimethyl-3,7-dimethylnonane-dioate. This prevails much over the typical cationic methoxyaddition. The reactive primary carboxy group formed allows for e.g. the preparation of the high molecular weight novel polyester poly[3,7-dimethylnonanediyl-3,7-dimethylnonanedioate].

 Please wait while we load your content...
Please wait while we load your content...