Catalytic depolymerization of alkali lignin in subcritical water: influence of formic acid and Pd/C catalyst on the yields of liquid monomeric aromatic products†
Abstract
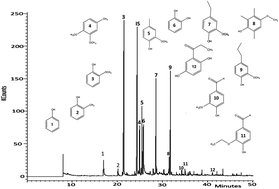
Alkali lignin was subjected to depolymerization in subcritical water at 265 °C, 6.5 MPa for reaction times between 1–6 h in a batch reactor and in the presence of formic acid (FA) and Pd/C catalyst. The oil products were extracted into diethyl ether and contained >90% of single-ring phenolic compounds. The reaction of lignin in subcritical water alone yielded 22.3 wt% oil containing 56% guaiacol as the main product. A maximum oil yield of 33.1 wt% was obtained when the lignin was reacted in the presence of formic acid alone. In the presence of FA, catechol became the predominant compound, with more than 80% of the ether extract after 6 h. The conversion of guaiacol to catechol in the presence of formic acid suggested the hydrolysis of O–CH3 ether bonds. In addition, the yields of alkyl guaiacols increased in the presence of FA. The use of 5 wt% Pd/C catalyst with FA slightly decreased the yields of oil products but led to compounds indicative of hydrogenolysis of aryl–O ether bonds as well as hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.
C bonds.

 Please wait while we load your content...
Please wait while we load your content...