t-BuXPhos: a highly efficient ligand for Buchwald–Hartwig coupling in water†
Abstract
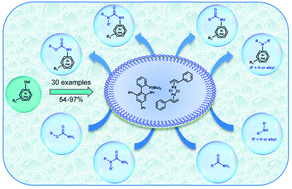
An efficient and versatile ‘green’ catalytic system for the Buchwald–Hartwig cross-coupling reaction in water is reported. In an aqueous micellar medium, the combination of t-BuXPhos with [(cinnamyl)PdCl]2 showed excellent performance for coupling arylbromides or chlorides with a large set of amines, amides, ureas and carbamates. The method is functional-group tolerant, proceeds smoothly (30 to 50 °C) and provides rapid access to the target compounds in good to excellent isolated yields. When applied to the synthesis of a known NaV1.8 modulator, this method led to a significant improvement of the E-factor in comparison with classical organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...