Boronate microparticle-supported nano-palladium and nano-gold catalysts for chemoselective hydrogenation of cinnamaldehyde in environmentally preferable solvents†
Abstract
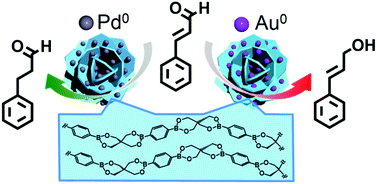
Dispersible self-assembled boronate polymers can serve as support materials for metal nanoparticle catalysts. As a proof-of-concept, catalytic systems for the chemoselective hydrogenation of cinnamaldehyde (CA) were prepared by the NaBH4 reduction of PdCl42− and AuCl4− in methanol, in the presence of polyethyleneimine (PEI)-coated boronate particles (BPs) composed of polymeric 3-benzo-2,4,8,10-tetraoxa-3,9-diboraspiro[5.5]undecane. The Pd-deposited BP (Pd/BP) showed highly selective catalytic activity for the hydrogenation of CA to hydrocinnamaldehyde (HCA) under 0.1 MPa of H2 at 25 °C in environmentally preferable solvents such as water and methanol. Of particular note is the recyclability of the hydrogenation catalyst in methanol; repeated 4 h reactions afforded HCA in >90% selectivity with a reaction conversion of 100%. On another front, when the corresponding Au catalyst (Au/BP) was used in the hydrogenation in water, a favorable selective reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to cinnamyl alcohol (CAL) (selectivity ≈ 80%) was obtained with 78% reaction conversion under 0.8 MPa of H2 at 80 °C for 12 h. The potential use of BP-supported metal nanoparticles as green catalysts is discussed.
O to cinnamyl alcohol (CAL) (selectivity ≈ 80%) was obtained with 78% reaction conversion under 0.8 MPa of H2 at 80 °C for 12 h. The potential use of BP-supported metal nanoparticles as green catalysts is discussed.
- This article is part of the themed collection: In celebration of Seiji Shinkai's 70th Birthday

 Please wait while we load your content...
Please wait while we load your content...