Synthesis of 2-substituted pyrimidines and benzoxazoles via a visible-light-driven organocatalytic aerobic oxidation: enhancement of the reaction rate and selectivity by a base†
Abstract
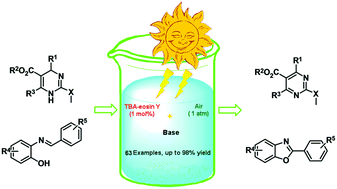
An efficient visible-light-driven photocatalytic oxidation of various 2-substituted dihydropyrimidines and phenolic imines has been achieved using an organic photocatalyst eosin Y bis(tetrabutyl ammonium salt) (TBA-eosin Y) and inexpensive oxidant molecular oxygen. With the aid of a base, significantly enhanced photoinduced electron transfer from substrates dihydropyrimidines or phenolic imines to the excited state of TBA-eosin Y has enabled the aerobic oxidation to yield 2-(methylthio)pyrimidines or 2-arylbenzoxazoles selectively.

 Please wait while we load your content...
Please wait while we load your content...