Efficient biocatalytic processes for highly valuable terminally phosphorylated C5 to C9 d-ketoses†
Abstract
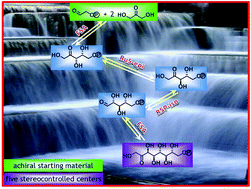
A green enzymatic strategy for the synthesis of terminally phosphorylated C5 to C9 naturally occurring D-ketose phosphates and analogues was developed using D-fructose-6-phosphate aldolase (FSA) as a catalyst. This enzyme has stereoselectively catalysed aldol reactions between glycolaldehyde phosphate or ribose-5-phosphate as an acceptor substrate and dihydroxyacetone, hydroxyacetone or hydroxybutanone as a donor. Furthermore, D-glycero-D-altro-2-octulose 8-phosphate was obtained using a straightforward one-pot domino biocatalytic system involving FSA, ribulose-5-phosphate epimerase and ribose-5-phosphate isomerase controlling five contiguous asymmetric centres and starting from achiral material.

 Please wait while we load your content...
Please wait while we load your content...