Barium sulphate catalyzed dehydration of lactic acid to acrylic acid†
Abstract
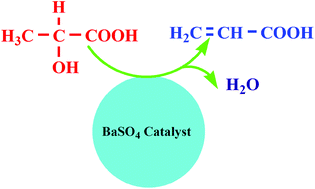
Dehydration of lactic acid was performed over various metal sulphates. BaSO4 was found to show an efficient activity for dehydration of lactic acid to acrylic acid due to the moderate acidity on its surface. Under the optimal conditions, 99.8% lactic acid conversion and 74.0% acrylic acid selectivity were achieved over the BaSO4 catalyst.

 Please wait while we load your content...
Please wait while we load your content...