Aerobic oxidation of isosorbide and isomannide employing TEMPO/laccase†
Abstract
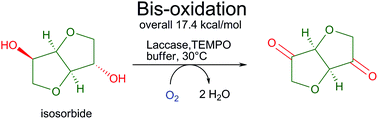
The oxidation of the renewable diols isosorbide and isomannide was successfully achieved using a TEMPO/laccase system. Furthermore, various TEMPO-derivatives were tested leading to conversions of up to >99% for the oxidation of isosorbide, isomannide, indanol and a halohydrin to the corresponding ketone.

 Please wait while we load your content...
Please wait while we load your content...