Efficient scavenging of β-carotene radical cations by antiinflammatory salicylates
Abstract
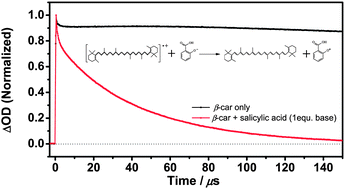
The radical cation generated during photobleaching of β-carotene is scavenged efficiently by the anion of methyl salicylate from wintergreen oil in a second-order reaction approaching the diffusion limit with k2 = 3.2 × 109 L mol−1 s−1 in 9 : 1 v/v chloroform–methanol at 23 °C, less efficiently by the anion of salicylic acid with 2.2 × 108 L mol−1 s−1, but still of possible importance for light-exposed tissue. Surprisingly, acetylsalicylate, the aspirin anion, reacts with an intermediate rate in a reaction assigned to the anion of the mixed acetic–salicylic acid anhydride formed through base induced rearrangements. The relative scavenging rate of the β-carotene radical cation by the three salicylates is supported by DFT-calculations.

 Please wait while we load your content...
Please wait while we load your content...