Spontaneous supersaturation of calcium d-gluconate during isothermal dissolution of calcium l-lactate in aqueous sodium d-gluconate
Abstract
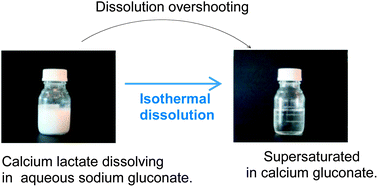
Continuing dissolution of solid calcium L-lactate pentahydrate in saturated aqueous solutions following addition of solid sodium D-gluconate corresponding to a gluconate/lactate ratio around three was found to result in homogeneous solutions supersaturated with calcium D-gluconate by a factor of seven, from which calcium D-gluconate monohydrate precipitated only slowly. In contrast, dissolution of calcium D-gluconate monohydrate by sodium L-lactate in aqueous solution with the reverse lactate/gluconate ratio also around three did not result in similar homogeneous solutions on the route to solid calcium L-lactate pentahydrate. This increasing supersaturation of calcium D-gluconate during dissolution of calcium L-lactate in aqueous sodium D-gluconate may enhance calcium bioavailability. The dissolution overshooting depends on competitive kinetics and is also of interest in modeling biomineralization and in designing novel food products with increased calcium bioavailability.

 Please wait while we load your content...
Please wait while we load your content...