Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide†
Abstract
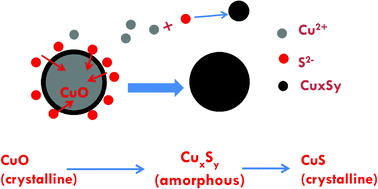
Many nanoparticles (NPs) are transformed in the environment, and the properties of the transformed materials must be determined to accurately assess their environmental risk. Sulfidation is expected to alter the speciation and properties of CuO NPs significantly. Here, commercially available 40 nm CuO NPs were characterized and sulfidized in water by inorganic sulfide, and the properties of the resulting products were determined. X-ray absorption spectroscopy, X-ray diffraction, and transmission electron microscopy indicate that CuO (tenorite) is sulfidized by inorganic sulfide to several copper sulfide (CuxSy) species including crystalline CuS (covellite), and amorphous (CuxSy) species at ambient temperature. Some Cu(II) was reduced to Cu(I) during sulfidation, coupled with sulfide oxidation to sulfate, resulting in the formation of small amounts of several copper sulfate hydroxide species as well. The extent of sulfidation depends on the sulfide to CuO molar concentration ratio used. At the highest S/Cu molar ratio of 2.16, 100% sulfidation was not reached in 7 days, as evidenced by the persistence of small amounts of CuO in the NPs. Sulfidation increased the fraction of copper passing a 3 kDa MWCO filter representing soluble forms of Cu and any small CuxSy clusters compared to the pristine CuO NPs at environmentally relevant neutral pH. This high solubility is a result of oxidative dissolution of CuxSy, formation of relatively more soluble copper sulfate hydroxides, and the formation of small CuS nanoclusters that pass the 3 kDa MWCO filter. These findings suggest that sulfidation of CuO may increase its apparent solubility and resulting bioavailability and eco-toxicity attributed to toxic Cu2+.
- This article is part of the themed collection: Environmental Science: Nano 2014 Most Accessed Articles

 Please wait while we load your content...
Please wait while we load your content...