Influences of different environmental parameters on the sorption of trivalent metal ions on bentonite: batch sorption, fluorescence, EXAFS and EPR studies†
Abstract
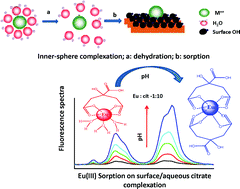
The presence of long-lived radionuclides in natural aquatic systems is of great environmental concern in view of their possible migration into biospheres of mankind. Trivalent actinides such as 241/243Am can contribute a great deal to radioactivity for several thousand years. This migration is significantly influenced by various factors such as pH, complexing ions present in aquatic environments, and the sorption of species involving radionuclides by sediments around water bodies. Clay minerals such as bentonite are known to be highly efficient in radionuclide retention and hence are suitable candidates for backfill materials. This study presents experimental results on the interaction of Eu(III) and Gd(III) (chemical analogs of Am(III) and Cm(III)) with bentonite clay under varying experimental conditions of contact time, pH, and the presence of complexing anions such as humic acid (HA) and citric acid (cit). The sorption of HA on bentonite decreased with increasing the pH from 2 to 8, which was attributed to electrostatic interactions between HA and the bentonite surfaces. The sorption of Eu(III) on bentonite colloids showed marginal variation with pH (>95%). However, a decrease in Eu(III) sorption was observed in the presence of HA beyond pH 5 due to the increased aqueous complexation of Eu(III) with deprotonated HA in the aqueous phase. The complexation of Eu(III) with citrate ions was studied using Time Resolved Laser induced Fluorescence Spectroscopy (TRLFS) to explain the sorption data. Extended X-ray absorption fine structure (EXAFS) and electron paramagnetic resonance (EPR) investigations were carried out to understand the local chemical environment surrounding Eu(III) and Gd(III) (EPR probe) sorbed on bentonite under different experimental conditions. Surface complexation modelling shows the predominant formation of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) XOEu+2 (silanol) up to pH < 7, and beyond which
XOEu+2 (silanol) up to pH < 7, and beyond which ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) YOEu(OH)+ (aluminol) is responsible for the quantitative sorption of Eu(III) onto bentonite in the studied pH range.
YOEu(OH)+ (aluminol) is responsible for the quantitative sorption of Eu(III) onto bentonite in the studied pH range.

 Please wait while we load your content...
Please wait while we load your content...