Kinetics of heterogeneous reactions of ozone with representative PAHs and an alkene at the air–ice interface at 258 and 188 K†
Abstract
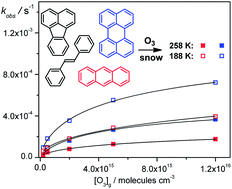
The kinetics of the reaction of an alkene (E-stilbene) and three polycyclic aromatic hydrocarbons (perylene, anthracene and fluoranthene), as examples of environmental pollutants, with ozone on the surface of ice grains (also called “artificial snow”), produced by shock-freezing of aqueous solutions, was studied at submonolayer pollutant coverages (c = 1.5 × 10−8 to 3 × 10−10 mol kg−1) and two different temperatures (258 and 188 K). This work supports and extends our previous discovery of a remarkable increase in the apparent ozonation rates with decreasing temperature. The ozonation kinetic results were evaluated using the Langmuir–Hinshelwood model and, in one case, the Eley–Rideal kinetic model. It is shown that the apparent rate enhancement is related to the specific nature of the ice surface at different temperatures, which influences the availability of contaminants to gaseous ozone, and to inherent reactivities of the contaminants. The maximum pseudofirst-order rate constants and the lifetimes of the studied compounds are provided. At a typical atmospheric ozone concentration in polar areas (50 ppbv), the lifetimes were estimated to be on the order of hours (258 K) or tens of minutes (188 K) for alkenes, and hundreds (258 K) or tens (188 K) of days for PAHs, thus approximately of the same magnitude or longer than those found for the gas-phase reactions. We imply that this rate enhancement at lower temperatures is a general phenomenon, and we provide data to implement heterogeneous reactions in snow in models that predict the extent of chemical reactions occurring in cold environments.
- This article is part of the themed collection: Aquatic photochemistry

 Please wait while we load your content...
Please wait while we load your content...