Organo-sulfur molecules enable iron-based battery electrodes to meet the challenges of large-scale electrical energy storage†
Abstract
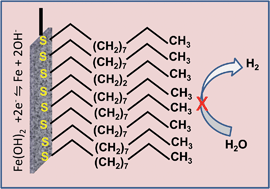
Rechargeable iron–air and nickel–iron batteries are attractive as sustainable and inexpensive solutions for large-scale electrical energy storage because of the global abundance and eco-friendliness of iron, and the robustness of iron-based batteries to extended cycling. Despite these advantages, the commercial use of iron-based batteries has been limited by their low charging efficiency. This limitation arises from the iron electrodes evolving hydrogen extensively during charging. The total suppression of hydrogen evolution has been a significant challenge. We have found that organo-sulfur compounds with various structural motifs (linear and cyclic thiols, dithiols, thioethers and aromatic thiols) when added in milli-molar concentration to the aqueous alkaline electrolyte, reduce the hydrogen evolution rate by 90%. These organo-sulfur compounds form strongly adsorbed layers on the iron electrode and block the electrochemical process of hydrogen evolution. The charge-transfer resistance and double-layer capacitance of the iron/electrolyte interface confirm that the extent of suppression of hydrogen evolution depends on the degree of surface coverage and the molecular structure of the organo-sulfur compound. An unanticipated electrochemical effect of the adsorption of organo-sulfur molecules is “de-passivation” that allows the iron electrode to be discharged at high current values. The strongly adsorbed organo-sulfur compounds were also found to resist electro-oxidation even at the positive electrode potentials at which oxygen evolution can occur. Through testing on practical rechargeable battery electrodes we have verified the substantial improvements to the efficiency during charging and the increased capability to discharge at high rates. We expect these performance advances to enable the design of efficient, inexpensive and eco-friendly iron-based batteries for large-scale electrical energy storage.

 Please wait while we load your content...
Please wait while we load your content...