Titanium incorporation into hematite photoelectrodes: theoretical considerations and experimental observations
Abstract
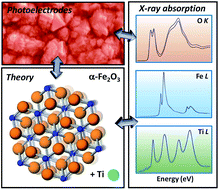
A theoretical and experimental perspective on the role of titanium impurities in hematite (α-Fe2O3) nanostructured photoelectrodes for solar fuel synthesis devices is provided. Titanium incorporation is a known correlate to efficiency enhancement in α-Fe2O3 photoanodes for solar water oxidation; here the relevant literature and the latest advances are presented and various proposed mechanisms for enhancement are contrasted. Available experimental evidence suggests that Ti incorporation increases net electron carrier concentrations in electrodes, most likely to the extent that (synthesis-dependent) charge compensating cation vacancies are not present. However, electron conductivity increases alone cannot quantitatively account for the large associated photoelectrochemical performance enhancements. The magnitudes of the effects of Ti incorporation on electronic and magnetic properties appear to be highly synthesis-dependent, which has made difficult the development of consistent and general mechanisms explaining experimental and theoretical observations. In this context, we consider how the electronic structure correlates with Ti impurity incorporation in α-Fe2O3 from the perspective of synchrotron-based soft X-ray absorption spectroscopy measurements. Measurements are performed on sets of electrodes fabricated by five relevant and unrelated chemical and physical techniques. The effects of titanium impurities are reflected in the electronic structure through several universally observed spectral characteristics, irrespective of the synthesis techniques. Absorption spectra at the oxygen K-edge show that Ti incorporation is associated with new oxygen 2p-hybridized states, overlapping with and distorting the known unoccupied Fe 3d–O 2p band of α-Fe2O3. This is an indication of mixing of Ti s and d states in the conduction band of α-Fe2O3. A comparison of spectra obtained with electron and photon detection shows that the effects of Ti incorporation on the conduction band are more pronounced in the near-surface region. Titanium L2,3-edge absorption spectra show that titanium is incorporated into α-Fe2O3 as Ti4+ by all fabrication methods, with no long-range titania order detected. Iron L2,3-edge absorption spectra indicate that Ti incorporation is not associated with the formation of any significant concentrations of Fe2+, an observation common to many prior studies on this material system.

 Please wait while we load your content...
Please wait while we load your content...