Constructing ionic highway in alkaline polymer electrolytes†
Abstract
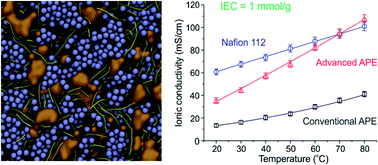
Alkaline polymer electrolytes (APEs) are an emerging material that enables the use of nonprecious-metal catalysts in electrochemical energy technology, such as fuel cell and water electrolysis. Yet the OH− conduction in APE has been of much lower efficiency than the H+ conduction in its acidic counterpart (typically Nafion), leading to a large dissipative loss in energy conversion applications. Here we report that, by properly constructing ion-aggregating structures in APE, a OH− conducting highway can be built, such that the OH− conduction in APE becomes as efficient as the H+ conduction in Nafion (greater than 0.1 S cm−1 at 80 °C under moderate ion-exchange capacity 1.0 mmol g−1). The optimal approach to constructing such an ionic highway is first screened computationally using coarse-grained molecular dynamics (CGMD) simulations, and then implemented experimentally based on a quaternary ammonia polysulfone (QAPS) model system. The resulting ordered structure of ion assembly has been unambiguously revealed by both the theoretically calculated structure factor and experimental results of TEM and SAXS. These findings have not only furthered our understanding about the ionic channels in APE, but also provided a general strategy for the rational design of polymer electrolytes.

 Please wait while we load your content...
Please wait while we load your content...