Charge transfer from cobaltammine complex cations to metal fluoride anions in molecular solids with novel photoelectronic effects (metal: zirconium, titanium)†
Abstract
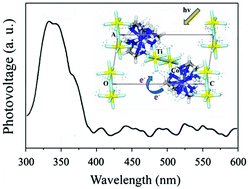
[Co(en)3](Ti2F11) containing H-bonded assembly of a discrete cobaltammine complex cation and titanium fluoride anion was successfully synthesized under solvothermal conditions. A novel photoelectronic effect was observed in the near-UV region. By extending our understanding of this compound, taking into account our previously reported three cobalt complex-containing zirconium fluorides, the mechanism of photoelectronic effects from these molecular solids was determined by investigating the relative work function for their component species with the help of a Scanning Kelvin probe. The results suggest that the charge transfer from the excited cobaltammine complex cations to the metal fluoride anions as a result of the cooperative behaviors might occur upon illumination, which is responsible for these novel photoelectronic effects.

 Please wait while we load your content...
Please wait while we load your content...