Synthesis and thermal decomposition of a pyridylene-bridged bis-β-diketiminate magnesium hydride cluster†
Abstract
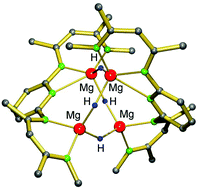
Reaction of PYR-(MgnBu)2, in which PYR is 2,6-[(DIPP)NC(Me)CHC(Me)N-]2-pyridine and DIPP is 2,6-iPr2-phenyl, with (DIPP)NH2BH3 gave PYR-[MgNH(DIPP)BH3]2 (56%) which was characterized by crystal structure determination. Addition of THF resulted in β-H elimination and formation of PYR-[MgNH(DIPP)BH3](MgH)·THF (57%), likewise characterized by crystal structure determination. Conversion of the second amidoborane anion in H− could not be achieved. Reaction of PYR-(MgnBu)2 with PhSiH3 gave PYR-(MgH)2, which crystallized as a dimer. The structure of [PYR-(MgH)2]2 shows an 8-membered ring of Mg2+ and H− ions. Thermal decomposition at 130 °C releases one equivalent of H2, i.e. 50% of the expected value. Nucleophilic attack at the para-position and reduction of the pyridylene bridge might explain reduced H2 release.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...