DFT study on the reaction mechanism of the ring closing enyne metathesis (RCEYM) catalyzed by molybdenum alkylidene complexes†
Abstract
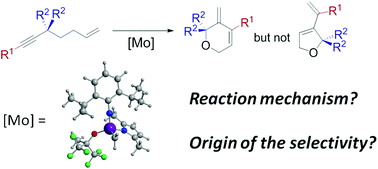
The ring closing enyne metathesis reaction (RCEYM) catalyzed by molybdenum based monoalkoxy pyrrolyl Schrock type catalysts has been studied by means of DFT (B3LYP-D) calculations. The two potential active alkylidene species as well as the three proposed reaction mechanisms (ene-then-yne, endo-yne-then-ene and exo-yne-then-ene) have been taken into account. Moreover, the influence on the exo- and endo- selectivity of the reactant substituents has also been explored. Results show that, in contrast to what is found for RCEYM processes catalyzed by Ru-based catalysts, the metallacyclobutene is a very short-living reaction intermediate that can be present in two isomeric forms (trigonal bipyramid (TBP) coordination around the metal center and square based pyramid (SPY) coordination). These two isomers are directly involved in the reaction mechanism, and the ring opening takes place from the SPY species. Moreover and regardless of the nature of the reacting metal-alkylidene, the yne-then-ene pathways (endo- or exo-) are computed to present significantly lower energy barriers than the ene-then-yne pathway and thus the latter is computed not to take place. Finally, the exo-/endo- selectivity is predicted to highly depend on the sterics of the two carbon ends of the alkyne fragment. In this way, the carbon bearing the largest group prefers to interact with the carbon end of the metal-alkylidene. This places the bulkiest groups as far away as possible from the metal fragment and overall leads to a generally lower energy barrier for the metallacyclobutene formation, the key step in defining the exo-/endo- selectivity.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...