Pyridine imines as ligands in luminescent iridium complexes†
Abstract
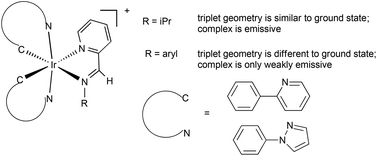
Biscyclometallated iridium complexes [Ir(ppz)2(X^Y)][PF6] (X^Y = pyridine imine) have been synthesised. The pyridineimine ligands are prepared in situ during the complexation. The complexes show room temperature emission between 640 and 780 nm in CH2Cl2 solution. The emission is red shifted compared with the analogous bipyridine complex [Ir(ppz)2(bipy)][PF6]. DFT calculations have been used to shed light on the influence of the imine substituent on the electrochemical and photochemical properties. In particular, the calculations suggests that there is a significant change in geometry between the ground state and the first triplet excited state for arylimines but not for alkylimines, leading to much weaker emission for the arylimine complexes. The work demonstrates that pyridineimines can be used as a substitute for bipyridines in luminescent iridium complexes.

 Please wait while we load your content...
Please wait while we load your content...