Meridional vs. facial coordination geometries of a dipodal ligand framework featuring a secondary coordination sphere†
Abstract
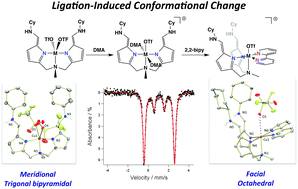
The synthesis of a novel dipodal ligand framework, H2[MeN(piCy)2], is summarized. Upon metalation with MCl2 salts (M = Fe, Cu), the ligand undergoes a conformational change, resulting in the formation of a trigonal bipyramidal metal center with a pseudoplanar, meridionally-bound ligand framework. This tautomerization positions pendant amines in the metal's secondary coordination sphere. Metalation with M(OTf)2 in coordinating solvent yields octahedral metal complexes, where two solvent molecules bind in the apical positions with one outer sphere counter ion. Reactivity of these complexes, (MeN(afaCy)2)M(X)2 (X = Cl, OTf), with 2,2′-bypyridine results in ligand reorganization, yielding a facial coordination geometry of the dipodal framework. The described complexes have been characterized by 1H NMR, EPR, IR, Mössbauer and electronic absorption spectroscopies as well as X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...