Zinc calixarene complexes for the ring opening polymerization of cyclic esters†
Abstract
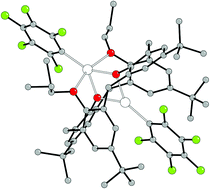
Reaction of Zn(C6F5)2·toluene (two equivalents) with 1,3-dipropoxy-p-tert-butyl-calix[4]arene (L1H2) led to the isolation of the complex [{Zn(C6F5)}2L1] (1), whilst similar use of Zn(Me)2 resulted in the known complex [{Zn(Me)}2L1] (2). Treatment of L1H2 with in situ prepared Zn{N(SiMe3)2}2 in refluxing toluene led to the isolation of the compound [(Na)ZnN(SiMe3)2L1] (3). The stepwise reaction of L1H2 and sodium hydride, followed by ZnCl2 and finally NaN(SiMe3)2 yielded the compound [Zn{N(SiMe3)2}2L1] (4). The reaction between three equivalents of Zn(C6F5)2·toluene and oxacalix[3]arene (L2H3) at room temperature formed the compound {[Zn(C6F5)]3L2} (5); heating of 5 in acetonitrile caused the ring opening of the parent oxacalix[3]arene and rearrangement to afford the complex [(L2)Zn6(C6F5)(R)(RH)OH]·5MeCN R = C6F5CH2-(p-tBuPhenolate-CH2OCH2–)2–p-tBuPhenolate-CH2O−)3− (6). The molecular structures of the new complexes 1, 3 and 6, together with that of the known complex 2, whose solid state structure has not previously been reported, have been determined. Compounds 1, 3–5 have been screened for the ring opening polymerization (ROP) of ε-caprolactone (ε-CL) and rac-lactide. Compounds featuring a Zn–C6F5 fragment were found to be poor ROP pre-catalysts as they did not react with benzyl alcohol to form an alkoxide. By contrast, compound 4, which contains a zinc silylamide linkage, was the most active of the zinc-based calix[4]arene compounds screened and was capable of ROP at ambient temperature with 65% conversion over 4 h.
- This article is part of the themed collection: The Carbon–Metal Bond and C–H Metalation

 Please wait while we load your content...
Please wait while we load your content...