Ferrocene-appended ligands for use in spin crossover-redox “hybrid” complexes of iron(ii) and cobalt(ii)†
Abstract
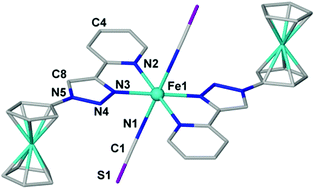
In a study of multifunctional (‘hybrid’) molecular materials, with one function being spin-crossover and the second being reversible redox behaviour, we describe ferrocene-appended ligands and their d6 and d7 complexes trans-[FeII(FTP)2(NCS)2] (1), [CoII(FTTP)2](ClO4)2·2(MeCN) (2) and [FeII(FTTP)2](ClO4)2·Et2O (3) (where FTP = 4-(2-pyridyl)-1H-1,2,3-triazol-1-ylferrocene and FTTP = 4′-ferrocenyl-2,2′:6′,2′′-terpyridine). The structures, magnetism and solution electrochemistry are described. Complex 1 remains high-spin, 2 displays gradual, incomplete spin crossover and 3 remains low-spin between 350–2 K. The electrochemical results show that one-electron oxidations at the ferrocene group, located external to the coordination site, occur at more positive potentials than the ‘inner’ MII/III couple in 1 and 2, but not in 3, and this has implications for retaining and influencing spin transitions at the MII centres, in future.


 Please wait while we load your content...
Please wait while we load your content...