Molecular excitons in a copper azadipyrrin complex†
Abstract
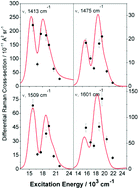
Exciton coupling is investigated in a copper azadipyrrin complex, Cu(L-aza)2. Exciton coupling in Cu(L-aza)2 assuming a single π–π* state on the L-aza ligand fails to account for the electronic structure of Cu(L-aza)2, which displays two almost equal intensity transitions at 15 600 cm−1 and 17 690 cm−1. TD-UB3LYP/6-31G(d) calculations suggest multiple π–π* transitions for the L-aza ligands and simple vector addition of the transition dipoles predicts two nearly orthogonal co-planar excitonic transitions that correctly reproduce the absorption band profile. Empirical modelling of absolute resonance Raman intensities using wavepacket dynamics confirms Cu(L-aza)2 has two equal intensity orthogonal exciton transitions. The phenyl substituents at the α- and γ-positions of the pyrrole rings play a central role in determining the orientation of the transition dipoles. Consequently the π–π* transitions for the L-aza ligands are oriented towards the substituent groups and are not in the plane of the pyrrole rings. Mode displacements in the Franck–Condon (FC) region obtained from the wavepacket model suggest that pyrrole ring and phenyl modes control the exciton FC dynamics. Our results suggest that Cu(L-aza)2 is an ideal model for theoretical, computational and experimental investigations of molecular excitons in molecular systems.
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States

 Please wait while we load your content...
Please wait while we load your content...