Synthesis and stability of Li/Cl carbenoids based on bis(iminophosphoryl)methanes†
Abstract
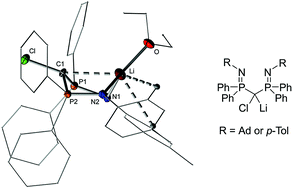
The synthesis and stability of two bis(iminophosphoryl) substituted lithium chloride carbenoids with different N-substituents (tolyl and adamantyl) were examined. Their preparation was accomplished via the mild oxidation of the corresponding dilithio bis(iminophosphoryl) methandiides, BIPMTol and BIPMAd, which are easily accessible by direct double deprotonation. In the case of the adamantyl substituted system the alternative preparation method via lithiation of the chlorinated precursor was found to be more selective. Here, the chlorinated precursor turned out to be highly CH acidic. Intramolecular deprotonation by the imino moiety results in the formation of the NH tautomer with a carbanionic centre as the most stable isomer. The prepared carbenoids are stable at room temperature in the solid state. Yet, in solution the stability was found to depend on the substituent bound at the imine nitrogen atom. As such, the tolyl substituted system was fairly stable in ether and hydrocarbon solvents, thus allowing crystallization. In contrast, the adamantyl derivative exhibits limited stability in ether solvents at room temperature. Decomposition resulted in LiCl elimination and transfer of the imino moiety to the carbenoid carbon atom.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...