Protonolysis and thermolysis reactions of functionalised NHC–carbene boranes and borates†
Abstract
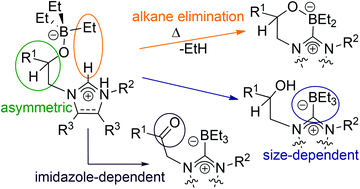
A set of β-ketoimidazolium and β-ketoimidazolinium salts of the general formula [R1C(O)CH2{CH[NCR3CR3N(R2)]}]X (R1 = tBu, naphth; R2 = iPr, Mes, tBu; R3 = H, Me, (H)2; X = Cl, Br) show contrasting reactivity with superhydride bases MHBEt3; two are reduced to chiral β-alcohol carbene–boranes R1CH(OH)CH2{C(BEt3)[NCR3CR3N(R2)]} 2 (R1 = tBu; R2 = iPr, Mes; R3 = H), two with bulky R2 substituents are reduced to chiral β-borate imidazolium salts [R1CH(OBEt3)CH2{CH[NCR3CR3N(R2)]}]X 3 (R1 = tBu, naphth; R2 = Mes, tBu; R3 = H, Me; X = Cl, Br), and the two saturated heterocycle derivatives remain unreduced but form carbene–borane adducts R1C(O)CH2{C(BEt3)[NCR3CR3N(R2)]} 4 (R1 = tBu, naphth; R2 = Mes; R3 = (H)2). Heating solutions of the imidazolium borates 3 results in the elimination of ethane, in the first example of organic borates functioning as Brønsted bases and forming carbene boranes R1CH(OBEt2)CH2{C[NCR3CR3N(R2)]} 5 (R1 = naphth; R2 = Mes; R3 = Me). The ‘abnormal’ carbene borane of the form 2 R1CH(OH)CH2{CH[NC(BEt3)CR3N(R2)]} (R1 = tBu; R2 = tBu; R3 = H), is also accessible by thermolysis of 3, suggesting that the carbene–borane alcohol is a more thermodynamically stable combination than the zwitterionic imidazolium borate. High-temperature thermolysis also can result in complete cleavage of the alcohol arm, eliminating tert-butyloxirane and forming the B–N bound imidazolium borate 7. The strong dependence of reaction products on the steric and electronic properties of each imidazole precursor molecule is discussed.

 Please wait while we load your content...
Please wait while we load your content...