Significantly enhanced dehydrogenation properties of calcium borohydride combined with urea†
Abstract
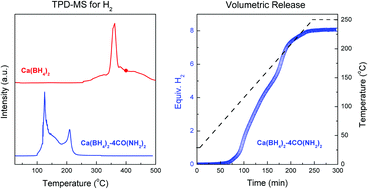
The interaction of [BHx]- and [NHx]-containing species gives rise to molecular hydrogen and the establishment of the B–N bond. Up to now, metal amides and ammonia are the commonly used [NHx] sources. Herein, urea, an organic carbonyl diamide, was used to react with Ca(BH4)2. A new type of complex hydride Ca(BH4)2·4CO(NH2)2 was synthesized with release of ca. 5.2 wt% hydrogen below 250 °C.

 Please wait while we load your content...
Please wait while we load your content...