Structural roles of amphiphilic peptide tails on silica biomineralization†
Abstract
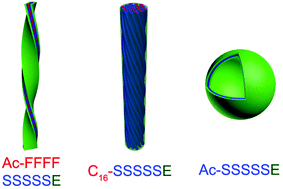
De novo synthesized amphiphilic peptides can be easily designed to form various nanostructures. Natural biomineralization creates the most intricately stunning inorganic structures, such as diatoms and shells, in which peptides play an important role. Here, we present the biomineralization of three designed amphiphilic peptides, which have different types of hydrophobic tails. By changing the hydrophobic tails from a phenylalanine-serine tail to an alkyl-serine tail or a serine-only tail, the conformations of peptides varied from type II β-turn to α-helix or random coil, which gave rise to the silica biomineralization nanostructures with nanoribbons, nanofibers and hollow nanospheres, respectively. Figuring out the structural roles of hydrophobic tails of amphiphilic peptides can improve strategies toward the bottom-up synthesis of nanomaterials as well as peptide scaffold engineering.

 Please wait while we load your content...
Please wait while we load your content...