Ferrocenyl-l-amino acid copper(ii) complexes showing remarkable photo-induced anticancer activity in visible light†
Abstract
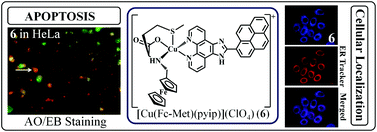
Ferrocene-conjugated copper(II) complexes [Cu(Fc-aa)(aip)](ClO4) (1–3) and [Cu(Fc-aa)(pyip)](ClO4) (4–6) of L-amino acid reduced Schiff bases (Fc-aa), 2-(9-anthryl)-1H-imidazo[4,5-f][1,10]phenanthroline (aip) and 2-(1-pyrenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (pyip), where Fc-aa is ferrocenylmethyl-L-tyrosine (Fc-Tyr in 1, 4), ferrocenylmethyl-L-tryptophan (Fc-Trp in 2, 5) and ferrocenylmethyl-L-methionine (Fc-Met in 3, 6), were prepared and characterized, and their photocytotoxicity was studied (Fc = ferrocenyl moiety). Phenyl analogues, viz. [Cu(Ph-Met)(aip)](ClO4) (7) and [Cu(Ph-Met)(pyip)](ClO4) (8), were prepared and used as control compounds. The bis-imidazophenanthroline copper(II) complexes, viz. [Cu(aip)2(NO3)](NO3) (9) and [Cu(pyip)2(NO3)](NO3) (10), were also prepared and used as controls. Complexes 1–6 having a redox inactive cooper(II) center showed the Fc+–Fc redox couple at ∼0.5 V vs. SCE in DMF–0.1 mol [Bun4N](ClO4). The copper(II)-based d–d band was observed near 600 nm in DMF–Tris-HCl buffer (1 : 1 v/v). The ferrocenyl complexes showed low dark toxicity, but remarkably high photocytotoxicity in human cervical HeLa and human breast adenocarcinoma MCF-7 cancer cells giving an excellent photo-dynamic effect while their phenyl analogues were inactive. The photo-exposure caused significant morphological changes in the cancer cells when compared to the non-irradiated ones. The photophysical processes were rationalized from the theoretical studies. Fluorescence microscopic images showed 3 and 6 localizing predominantly in the endoplasmic reticulum (ER) of the cancer cells, thus minimizing any undesirable effects involving nuclear DNA.

 Please wait while we load your content...
Please wait while we load your content...