The specificity of interaction of Zn2+, Ni2+ and Cu2+ ions with the histidine-rich domain of the TjZNT1 ZIP family transporter
Abstract
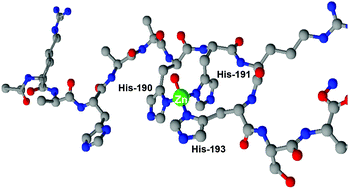
The Zrt/Irt-like protein (ZIP) family contributes to the metal homeostasis by regulating the transport of divalent metal cations such as Fe2+, Zn2+, Mn2+, Cd2+ and sometimes even Cu2+. Most ZIP members have a long variable loop between transmembrane domains (TMDs) III and IV; this region is predicted to be located in the cytoplasm and is postulated to be the metal ion binding site. In this study, we looked at the thermodynamic behavior and coordination chemistry of Zn2+, Ni2+ and Cu2+ complexes with the histidine-rich domain, Ac-(185)RAHAAHHRHSH(195)-NH2 (HRD), from the yeast TjZNT1 protein, located between TMDs III and IV. The sequence is conserved also in higher species like Thlaspi japonicum. The stability of complexes increases in the series Ni2+ < Zn2+ ≪ Cu2+. The geometry of complexes is very different for each metal and in the case of Zn2+ complexes, high specificity in binding is observed. Moreover, the stability of HRD–Cu2+ complexes was compared with the five His residues containing peptide from Hpn protein (Helicobacter pylori). The results suggest a high ability of HRD in the binding of all three studied metals.

 Please wait while we load your content...
Please wait while we load your content...