Lithium, sodium and potassium picolyl complexes: syntheses, structures and bonding†
Abstract
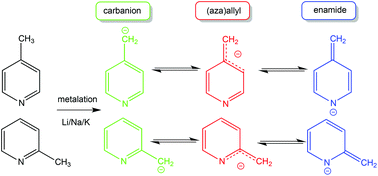
Synthetically important for introducing a picolyl scaffold into a molecular construction, alkali metallated picoline (methylpyridine) complexes are also interesting in their own right for the diversity of their ligand–metal bonding possibilities. Here the syntheses of seven new such complexes are reported: namely three 4-picoline derivatives 4-picLi·Me6TREN, 1, 4-picNa·Me6TREN, 2, and [4-picK·2(4-picH)]∞, 3; and four 2-picoline derivatives, 2-picLi·Me6TREN, 4, 2-picLi·PMDETA, 4′, 2-picNa·Me6TREN, 5, and [2-picK·PMDETA]2, 6′ [where pic = NC5H4(CH2); Me6TREN = tris(N,N-dimethyl-2-aminoethyl)amine, (Me2NCH2CH2)3N; PMDETA = N,N,N′,N′′,N′′-pentamethyldiethylenetriamine, (Me2NCH2CH2)2NMe]. X-ray crystallographic studies establish that the lighter alkali metal complexes 1, 2, 4′ and 5 adopt monomeric structures in contrast to the polymeric and dimeric arrangements adopted by potassium complexes 3 and 6′ respectively. All complexes have also been characterized by solution NMR spectroscopy (1H, 13C, and where relevant 7Li). This study represents the first example of sodium and potassium picolyl complexes to be isolated and characterized. DOSY (Diffusion-Ordered Spectroscopy) experiments performed on 4 and 4′ suggest both compounds retain their monomeric constitutions in C6D6 solution. Discussion focuses on the influence of the metal and neutral donor molecule on the structures and the nature of the ligand–metal (enamido versus aza-allylic) interactions.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...