Synthesis of new heteroleptic strontium complexes†
Abstract
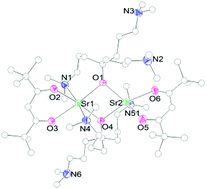
A series of heteroleptic strontium complexes (1–9) using a combination of different aminoalkoxides and 2,2,6,6-tetramethyl-3,5-heptanedionate (tmhd) were prepared to examine the effect of the bulkiness and coordination ability of the aminoalkoxide ligand in these complexes as well as potential strontium precursors. All complexes were characterized by FT-IR, FT-NMR, elemental analyses, and thermo-gravimetric (TG) analyses. The crystal structure analyses of 1, 2, 4, and 5 demonstrate their stability in the dimer form and the unwillingness of the strontium atom to form more than six coordination bonds in these complexes. The complex 5 shows an unusual picture: the existence of one hexa-coordinated and one penta-coordinated strontium atom side by side in its dimer structure. The introduction of ether groups as coordination sites in complexes 6–9 led to a decrease in steric hindrance which resulted in the formation of the complex 7 as a tetramer. The complex 7 shows a unique Sr4O4 cubane core where oxygen atoms undergo μ3-bridging between strontium atoms. The TG analyses show that the complexes exhibit a step-wise decomposition character, with the major mass losses in the region 150–400 °C.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...