Electrochemical reduction of dioxygen by copper complexes with pyridylalkylamine ligands dissolved in aqueous buffer solution: the relationship between activity and redox potential†
Abstract
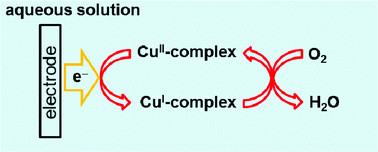
The CuII/CuI redox properties and electrochemical O2 reduction activity of a series of CuII-complexes with pyridylalkylamine ligands were investigated in a neutral buffer solution. The relationship between CuII/CuI redox properties and O2 reduction activity was clearly demonstrated by voltammetric analyses.

 Please wait while we load your content...
Please wait while we load your content...