Base-promoted aryl–bromine bond cleavage with cobalt(ii) porphyrins via a halogen atom transfer mechanism†
Abstract
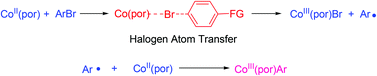
Aryl–bromine bonds are successfully cleaved by cobalt(II) porphyrins in basic media to give Co(por)Ar (por = porphyrin) in good yields. Mechanistic studies suggested that the aryl–bromine bond is cleaved through a halogen atom transfer mechanism, which is different from the aryl–halogen bond cleavage mechanism with other group 9 metalloporphyrins.

 Please wait while we load your content...
Please wait while we load your content...