NHC-coordinated silagermenylidene functionalized in allylic position and its behaviour as a ligand†
Abstract
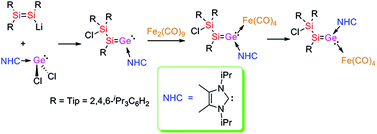
Vinylidenes are common in transition metal chemistry with catalytic applications in alkene and alkyne metathesis. We report here the isolation of a heavier analogue of vinylidene, an α-chlorosilyl functionalized silagermenylidene stabilized by an N-heterocyclic carbene (NHC). Silagermenylidene (Tip2Cl)Si-(Tip)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ge·NHCiPr2Me2 (4-E/Z; Tip = 2,4,6-iPr3C6H2; NHCiPr2Me2 = 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene) is available as an E/Z-equilibrium mixture from Tip2Si
Ge·NHCiPr2Me2 (4-E/Z; Tip = 2,4,6-iPr3C6H2; NHCiPr2Me2 = 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene) is available as an E/Z-equilibrium mixture from Tip2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(Tip)Li and NHCiPr2Me2·GeCl2. Reaction of 4-E/Z with Fe2(CO)9 affords a silagermenylidene Fe(CO)4 complex, which slowly isomerizes to its E-isomer at 25 °C. A rearranged Fe(CO)3 complex with an allylic SiGeSi ligand is obtained as a side product at 65 °C.
Si(Tip)Li and NHCiPr2Me2·GeCl2. Reaction of 4-E/Z with Fe2(CO)9 affords a silagermenylidene Fe(CO)4 complex, which slowly isomerizes to its E-isomer at 25 °C. A rearranged Fe(CO)3 complex with an allylic SiGeSi ligand is obtained as a side product at 65 °C.

 Please wait while we load your content...
Please wait while we load your content...