Diiron hexacarbonyl complexes as potential CO-RMs: CO-releasing initiated by a substitution reaction with cysteamine and structural correlation to the bridging linkage†
Abstract
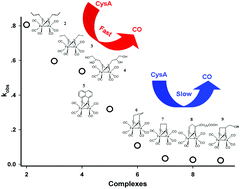
The CO-releasing behaviours of nine diiron carbonyl complexes (1–9) were examined via the substitution reaction of cysteamine (CysA), of which complex 4 was reported recently. These complexes fall into three categories, the diiron core bridged by two thiolates, a dithiolate and 1,8-naphthalene dithiolate. Our results reveal that the CO-releasing rates of these complexes are highly dependent on their structures. Complexes (2–4) bearing two monothiolates as their bridging linkage (“open” form) are more vulnerable to decomposition upon nucleophilic substitution reactions compared with complexes (6–9) that possess a dithiolate as their bridging linkage. When the bridging linkage lacks an electron-donating group (complex 1), the metal centre is less negatively charged as revealed by DFT calculation, and thus it exhibits fast substitution reaction with CysA to release CO. A linkage with conjugating nature (5) shows a similar effect because electron density on the metal centre decreases due to electron-density diverting from the metal centre into the naphthalene moiety. Kinetic analysis suggests that CO-releasing at the first stage of these complexes is a first-order reaction.

 Please wait while we load your content...
Please wait while we load your content...