Interaction of Cm(iii) and Am(iii) with human serum transferrin studied by time-resolved laser fluorescence and EXAFS spectroscopy
Abstract
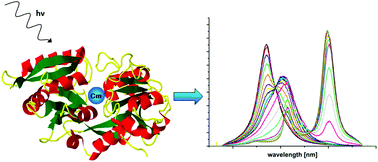
The complexation of Cm(III) with human serum transferrin was investigated in a pH range from 3.5 to 11.0 using time-resolved laser fluorescence spectroscopy (TRLFS). At pH ≥ 7.4 Cm(III) is incorporated at the Fe(III) binding site of transferrin whereas at lower pH a partially bound Cm(III) transferrin species is formed. At physiological temperature (310 K) at pH 7.4, about 70% of the partially bound and 30% of the incorporated Cm(III) transferrin species are present in solution. The Cm(III) results obtained by TRLFS are in very good agreement with Am(III) EXAFS results, confirming the incorporation of Am(III) at the Fe(III) binding site at pH 8.5.

 Please wait while we load your content...
Please wait while we load your content...