A novel type of coordination mode of chloranilic acid leading to the formation of polymeric coordination ribbon in the series of mixed-ligand copper(ii) complexes with 1,10-phenanthroline†
Abstract
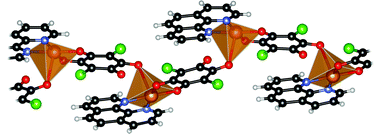
A series of four novel mixed-ligand complexes of copper(II) with 3,6-dichloro-2,6-dihydroxy-1,4-benzoquinone (chloranilic acid) and 1,10-phenanthroline was prepared and characterised by X-ray structure analysis and IR spectroscopy. Three complexes exhibit square-pyramidal coordination, whereas one exhibits octahedral coordination. The ligand 1,10-phenanthroline acts in a bidentate chelating mode with N,N-metal binding. The chloranilate dianion coordinates to the CuII atom in a terminal bidentate ortho-quinone-like mode, forming a mononuclear complex species. However, in one structure a novel type of coordination mode of chloranilate is observed. In addition to the bidentate mode, a monodentate bridging mode through a carboxy oxygen of a symmetry-related dianion leads to the formation of polymeric coordination ribbon. The crystal packing of penta-coordinated species, in addition to hydrogen bonding, involves less common stacking interactions of chelate rings with the π-systems of the ligands.

 Please wait while we load your content...
Please wait while we load your content...