Chiral phenoxyimino-amido aluminum complexes for the asymmetric cyanation of aldehydes†
Abstract
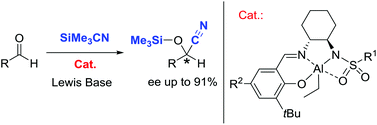
The reactivity of triethylaluminum towards salicylaldimine sulfonamides was probed, affording well-defined complexes through consecutive protonolysis of two Al–C bonds by the proligand. These complexes, when combined with an achiral anilinic N-oxide, catalyze the asymmetric addition of trimethylsilylcyanide to a wide range of aldehydes, with good activity and enantioselectivity (up to 91% ee). Insertion of the benzaldehyde substrate into the Al–N amido bond was observed, bringing elements for discussion around the nature of the actual active species.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...