CO2 selective 1D double chain dipyridyl-porphyrin based porous coordination polymers†
Abstract
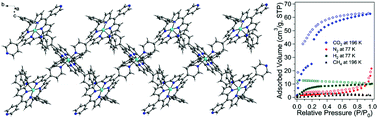
Thermal reactions of MnCl(DPyP) (DPyP = 5,15-di(4-pyridyl)-10,20-diphenylporphyrin) as a metalloligand with CoII and ZnII ions in dimethylformamide led to neutral one-dimensional (1D) double chain dipyridyl-porphyrin-based porous coordination polymers (PCPs), Co3(DPyP)3·4DMF (I) and Zn3(DPyP)3·2DMF·4H2O (II). Both PCPs were structurally characterized by X-ray crystallography. Particularly, the central MnIII ion in MnCl(DPyP) was transmetallated with CoII or ZnII ions and the central CoII or ZnII ions were further coordinated to pyridyl groups of neighboring M(DPyP) (M = Co or Zn) porphyrin complexes. PCPs I and II are isostructural and each 1D double chain interacts with another 1D double chain by multiple hydrogen bonding to stabilize the resulting framework. Therefore, solvent-free 1D double chain PCPs have permanent porosity, and the void volumes of the solvent-free I and II are calculated to be 22.6% and 23.0%, respectively. Gas sorption analysis indicated that I and II exhibited selective adsorption of CO2 at 196 K. Both PCPs exhibited much smaller sorption abilities for N2 (77 K), H2 (77 K), and CH4 (196 K) than CO2 (196 K). Both PCPs exhibited different PXRD patterns when dried at 373 K, which indicated that the framework transformation of the isostructural M3(DPyP)3 type of PCPs strongly depended on the type of central metal ions.

 Please wait while we load your content...
Please wait while we load your content...