Metal-assisted conversion of an N-ylide mesomeric betaine into its carbenic tautomer: generation of N-(fluoren-9-yl)imidazol-2-ylidene complexes†
Abstract
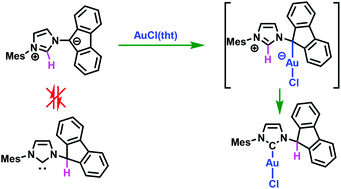
Whereas the N-ylide mesomeric betaine 2, consisting of a fluorenyl anion directly attached to an imidazolium ring, is not in equilibrium with its putative free N-(fluoren-9-yl)imidazol-2-ylidene tautomer, its reaction with a metallic centre induces its interconversion to yield the corresponding monoligated N-heterocyclic carbene complex (Au(I) and Rh(I)). Deprotonation of 2 and coordination to the RhI(COD) fragment allows the isolation of complex 7 displaying a rarely observed four-membered NHC-containing metallacycle and an enforced η1-fluorenyl ligand. Upon reaction with CpFe(CO)2I precursor, insertion of a carbonyl ligand into the Fe–fluorenyl bond occurs and yields the acyl–Fe complex 8.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...