Mechanism of tetrachloroplatinate(ii) oxidation by hydrogen peroxide in hydrochloric acid solution†
Abstract
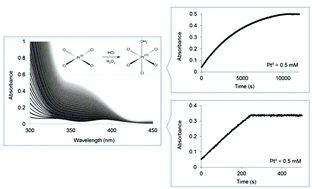
Oxidation of tetrachloroplatinate(II) by hydrogen peroxide in hydrochloric acid was studied by UV-Vis spectrophotometry. Oxidation takes place via two parallel reactions with hypochlorous acid and hydrogen peroxide, respectively, according to the overall rate law d[Pt(IV)]/dt = (k0 + kH2O2[Pt(II)])[H2O2]. For oxidation of [PtCl4]2− at relatively low concentrations, [PtCl4]2− ≪ 0.5 mM, hypochlorous acid formation is fast relative to the oxidation of [PtCl4]2− by hydrogen peroxide, as a result of the rate determining reaction H2O2 + H+ + Cl− → HOCl + H2O, resulting in a rate law d[Pt(IV)]/dt = k0[H2O2] with a value k0 = (8 ± 2) × 10−7 s−1 at 35 °C. For concentrations of [PtCl4]2− > 0.5 mM, oxidation by hydrogen peroxide becomes dominant, resulting in the pseudo-first order rate law d[Pt(IV)]/dt = kH2O2[Pt(II)][H2O2] with the value kH2O2 = (1.5 ± 0.1) × 10−2 M−1 s−1 at 35 °C. The final oxidation product is a mixture of [PtCl5(H2O)]− and [PtCl6]2−, with [PtCl6]2− formed as a result of [PtCl4]2− assisted chloride anation reactions.

 Please wait while we load your content...
Please wait while we load your content...